Article Outline
Figures and tables
Volume 5 Issue 1 > pp. 14-20 • doi: 10.15627/jd.2018.2
Exploring the Impact of Natural Light Exposure on Sleep of Healthy Older Adults: A Field Study
Mariëlle P. J. Aarts,a* Janny C. Stapel,b,c Toine A. M. C. Schoutens,d Joost van Hoofe,f
Author affiliations
a Department of the Built Environment, Eindhoven University of Technology, Eindhoven, The Netherlands
b Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, The Netherlands
c University of Oxford, Oxford, UK
d Light and Health Research Foundation - SOLG, Eindhoven, The Netherlands
e Faculty of Social Work & Education, The Hague University of Applied Sciences, The Hague, The Netherlands
f Department of Spatial Economy, Wrocław University of Environmental and Life Sciences, Wrocław, Poland
* Corresponding author. Tel.: +31 40 247 2900
M.P.J.Aarts@tue.nl (M. P. J. Aarts)
j.c.stapel@donders.ru.nl (J. C. Stapel)
info@SOLG.nl (T. A. M. C. Schoutensh)
j.vanhoof@hsh.nl (J. v. Hoof)
History: Received 21 February 2018 | Revised 16 April 2018 | Accepted 21 April 2018 | Published online 4 May 2018
Copyright: © 2018 The Author(s). Published by solarlits.com. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Citation: Mariëlle P. J. Aarts, Janny C. Stapel, Toine A. M. C. Schoutens, Joost van Hoof, Exploring the Impact of Natural Light Exposure on Sleep of Healthy Older Adults: A Field Study, Journal of Daylighting 5 (2018) 5-14 http://dx.doi.org/10.15627/jd.2018.2
Figures and tables
Abstract
Studies among people with dementia demonstrated that the sleep quality and rhythm improves significantly when people are exposed to ambient bright light. Since almost half of the healthy older people also indicate to suffer from chronic sleep disorders, the question arises whether ambient bright light can be beneficial to healthy older people. Particularly the effect on sleep/wake rhythm in relation to the exposure to natural light is the focus. It was hypothesised that the sleep quality would be worse in winter due to a lower daylight dose than in summer due to the lower illuminance and exposure duration. A field study was conducted to examine the relationship between daylight exposure and sleep quality in 14 healthy older adults living independently in their own dwellings in the Netherlands. All participants were asked to take part of the study both during the summer period as well as during the winter period. Therefore, they had to wear an actigraph for five consecutive days which measured sleep, activity and light exposure. Results confirmed that people were significantly longer exposed to high illumination levels (>1000 lx) in summer than in winter. Sleep quality measures, however, did not differ significantly between summer and winter. A significant, positive correlation was found between exposure duration to high illuminance from daylight during the day and the sleep efficiency the following night in summer, implying that being exposed to high illuminance for a longer time period has a positive effect on sleep efficiency for the individual data. There was also a tendency of less frequent napping in case of longer exposure duration to light for both seasons. Sleep quality does not differ between summer and winter but is related to the duration of the exposure to bright light the day prior to the night.
Keywords
Older people, Sleep, Daylight exposure, Outdoors
1. Introduction
Older adults are known to be worse sleepers than their younger counterparts. Epidemiological studies [1,2] show that 40-70% of older adults suffer from chronic sleep problems. Only a mere 20% claim to have no sleeping disturbances at all. Typical characteristics of sleeping problems are difficulty falling asleep, nocturnal sleep fragmentation, increased daytime sleepiness, and daytime napping. Apart from physiological causes, sleeping problems can stem from the social setting. From care homes for the aged it is known that older adults are minimally stimulated to be active during the day [3].
Hattar et al. [4] described a third kind of photo receptor in the eye, called intrinsically photosensitive retinal ganglion cells (ipRGCs). Berson et al. [5] discovered a previously unknown function of these cells in relation to phototransduction. The action spectrum of this photo receptor differs significantly from the other two known receptors for scotopic and photopic vision; rods and cones. These receptors all participate in mammalian circadian phototransduction; namely by transmitting signals to the suprachiasmatic nuclei (SCN), the location of the biological clock in the human brain. The SCN are capable of (i) generating near 24-hour cycles in cell-firing patterns, of (ii) synchronising these cycles with the environmental light/dark cycle, and of (iii) cyclic control of brain structures involved in body temperature and the release of hormones. The action spectrum for melatonin suppression shows a peak around 459-464 nm [6,7], indicating short wavelength sensitivity of the circadian system. With ageing, the 24-hour cycles or so-called circadian rhythms show changes. Age-related changes of the circadian timing system can be found in amplitude and period of the rhythms, as well as synchronisation with the environment and sensitivity to environmental clues; the so-called ‘Zeitgebers’ [8]. A decreased neuronal activity is a characteristic of the aged SCN themselves [9]. Moreover, the number of retinal ganglion cells may decrease with ageing [10].
In the SCN of older adults, the amount and synthesis of the main peptides that show a circadian rhythm, decreases. This could be an underlying cause of sleep-wake rhythm disturbances. The SCN are sensitive to a number of stimuli and have a certain flexibility that is preserved over the years. Therefore, the consequences of an unstable circadian pacemaker might be reversible for a certain period [11]. It is postulated that exposure to bright light can be used to treat some sleep disorders involving the timing and duration of sleep [12].This is supported by evidence that light is capable of restoring the circadian amplitude in sleep-wakefulness of older adults [13], and improve restless behaviour among institutionalised older adults with dementia [14]. Other studies also indicated that older adults, who were exposed longer to high light levels, show a better sleep-wake rhythm [15]. Herljevic et al. [16] showed diminished suppression of melatonin in women after menopause in comparison with younger people. This effect was ascribed to a number of ageing-related changes to the eye, including darkening of the lens and development of a yellow pigmentation, known to reduce the transmission of short wavelength light. Mishima et al. [17] showed that older adults are exposed to lower light levels than young adults. They also showed that insomniac older adults, when receiving a comparable amount of light as young people, regained a melatonin production comparable with the younger people in the study, implying that light contributes to increase the amplitude of the circadian rhythm. A study by Kirisoglu and Guilleminault [18] showed that sleeping problems in older adults aged 60 years and over can be lessened by light therapy in the morning (baseline and 3 and 6 months post-treatment design). A 45-minute exposure to illuminance levels of 10 000 lx emitted from a light box at a distance of 0.75 metres just after rising in the morning within 5 minutes of wake-up time substantially ameliorated sleep quality and lessened daytime fatigue, than did a daily 20-minute exposure of 10 000 lx.
Since humans have evolved under the influence of daylight and the natural light-dark cycle. People have developed a variety of physiological responses to the varied characteristics of daylight for example the reflex of changing pupil diameter under different light conditions. In addition, the human skin provides a layer of pigmentation to protect us from the high ultraviolet intensities when exposed to daylight almost every day. Our natural rhythm is to be active when it is daytime and to be inactive (sleep) during the night. Due to the development of electrical light sources, people are able to alter that active period so to be active during the naturally dark period of the day. Although many light characteristics can be modeled by electrical light sources, major differences still exist compared to daylight like:
- Daylight is dynamic, depending on the weather, location on earth, climate, time of day and time of year.
- The dynamics of the daylight consist of the illuminance level (spherical), the light spectrum (spherical), directionality of the light (direct sunlight and diffuse).
- Timing of the dynamics. Light fluctuations can range between milliseconds to years.
- Daylight consists of a much wider electromagnetic spectrum than only light. The most closely related are Ultra Violet and Infrared radiation.
- Daylight consists of all the different wavelengths making white light.
Therefore, it is assumed that exposure to natural light has an even stronger positive effect on older people and specifically the sleep/wake cycle. The literature review by Aries and colleagues [19] already demonstrated the difficulty to make a distinction between the actual physical phenomenon (radiation) and its related psychological aspects (view, sunlight, temperature). Therefore going outdoors and being exposed to extremely high illuminance levels are treated similar.
Only few studies reported the relation between natural light exposure and circadian health effects. One of the earliest studies on the influence of light on hormone secretion was done by Hollwich and Dieckhues in 1980 [20]. They concluded that blind and temporarily blind patients, due to cataract, have significant low levels of ACTH (Adreno CorticoTroop Hormone) and cortisol. After cataract extraction, the levels normalized again. They also found that people exposed to high illumination (3500 lx) with a very different spectral light composition than daylight showed stress-like levels of ACTH and cortisol. They suggest, to avoid mental and physical alterations, that the spectrum of electric lighting should be largely similar to that of natural light. In 1991, Baskett et al [21] studied the melatonin plasma profiles between older people who had not been exposed to natural light for more than six weeks due to hospitalization (n = 6) and healthy older people (n = 15). The hospital group had significantly higher daytime plasma melatonin levels, an earlier nocturnal rise and the timing of their secretory profiles was more variable. They concluded therefore that the currently used artificial and supplementary natural lighting may not be sufficient to suppress melatonin secretion adequately during daylight hours nor act efficiently to entrain day/night secretion of melatonin in a physiological circadian manner. These might result in mood and sleep disorders. A similar but larger cross-sectional study was performed in 2012 by Obayashi et al [22] among 192 older people individuals in Japan. The research question was whether daylight exposure would increase nocturnal melatonin secretion in an uncontrolled daily life setting. They found a positive correlation between duration of light exposure of >1000 lx on wrist level and urinary 6-sulfatoxymelatonin excretion during night time. An increase of 13% between 37 and 124 minutes (25th to 75th percentiles) exposure was found. Another non-lab study regarding the same topic [23] found no relation between light exposure and melatonin production but did find a positive correlation between daytime activity and melatonin production during the night. Since the subjects were all women in the age category between 18 and 62 years old, light effects were only revealed among the older people.
Tsuzuki and colleagues [24] examined the seasonal influence of light on sleep in eight healthy older men (> 60 years., mean age of 64 ± 1 yrs.) in the four seasons starting in spring. They showed that the time to wake-up in summer was significantly later than in the other seasons, also the number and duration of nocturnal awakenings where higher in summer. The results suggest that the sleep quality in summer is worst compared to the rest of the year. In their study they also show that being exposed to high lighting levels four hours before going to bed delays the bedtime and the wake-up time advances. These results were supported by the same research group [25]. They did a similar research in Japan among 19 older people (male / female = 13/ 6). The results show that the sleep latency, wake time, number of wake episodes, and activity index significantly increased in summer, compared to fall or winter. Furthermore, compared to fall and winter, the sleep time and the sleep efficiency index significantly decreased in summer. They also found a significant correlation between bedroom climate and the sleep parameters: wake episode, sleep efficiency index and wake after sleep onset. These results were only found in summer. The continuous light exposure was not reported.
Calkins and colleagues [26] conducted a pre-post quasi-experimental design on people with dementia in a nursing home, in order to answer the question whether time spent outdoor influences several psychophysical aspects of 17 older people with dementia. Results suggest that increased time spent outdoors resulted in a modest improvement in sleep efficiency of 10%, and mixed or immeasurable impact on agitation. Simular results were found by Gammack and Burke [27] where they examined the correlation between natural light exposure and self-reported sleep disturbances in a nursing home. The data suggest that natural sunlight exposure may improve some measures of subjective sleep quality but is not likely a panacea for poor sleep quality.
Natural light exposure is an inexpensive therapy that can be administered simultaneously to many individuals in an institutional setting. Resident resistance to outdoor activities, weather, and medical illness are potential limitations to this approach.
Of course, high light levels can be achieved by electrical lighting and might be more relevant to the lighting industry [28]. The aim of this study is to find out whether mobile and healthy older adults, with and without a history of sleeping problems, show a better sleep-wake pattern when exposed to high levels of natural, daylight (available in summer) compared to low intensity natural daylight (available in winter).
Although the minimum amount of light necessary for restoring sleep-wake rhythm is still unknown, the retinal light exposure of older adults is less than that of younger people, due to the aforementioned ageing of the eye [29].
Methodology
2.1. Experiment setting
A cohort of 14 subjects was used for this study (9 females, 5 males). The age of the participants ranged from 65 to 80 years (mean ± SD; 67 ± 4.1 years). The participants were all in good general health, mobile, and lived independently in their own dwelling. Half of the participants reported sleeping problems. This was confirmed by the results of the Pittsburgh Sleep Quality Index (PSQI) [30], which tended to overestimate the number of bad sleepers (5 ‘good-sleepers’ and 9 ‘bad-sleepers’). The median PSQI score at baseline was 6.5. There was no significant difference between summer and winter PSQI scores. No one reported to be using sleeping medication. The only reported eye disease was cataract. One person had been (successfully) operated and two still suffered from it. Sunglasses were never worn by nine people and three people always wore sunglasses when going outside. The remaining two occasionally wore sunglasses. The subjects with cataract occasionally wore sunglasses when they went outside. The combination of the two reduced the amount of light reaching the retina. Nevertheless, assumed was that the levels of light exposure were still above 1 000 lx vertically and therefore these people were not excluded from the research.
2.2. Materials
2.2.1. Actigraphy
The participants were asked to wear an actigraph combined with a calibrated light sensitive cell (Cambridge Neurotechnology Actiwatch, type Actiwatch-L) around the non-dominant wrist. The light sensitive cell was designed to measure up to 32 000 lx. A 15-second sampling interval was used meaning that a maximum of five consecutive days could be measured.
2.2.2. Sleep log
Participants were asked to keep a sleep log on (i) time they went to bed, (ii) how long it took to fall asleep, (iii) at what time participants woke up in the morning, (iv) at what time they got up, and (v) time of being awake / waking up during the night, for instance, to go to the toilet during the night. The registered data was used as input for the actigraphy analyses.
2.2.3. Daylight exposure
Participants were asked to register the time they spent outdoors (in open air). The subjects also had to write down whether or not the actigraph was covered by clothing, for instance a sleeve, or not worn at all. Those data were discarded from analysis, since the analyses were based on the actigraph data only. The time people spent outdoors was used to check the measured data of the actiwatch. Only one person reported that the device was once covered. In general, the device was only removed for a shower/swim and only for a maximum period between 20 to 60 minutes per day.
2.2.4. PSQI
The Pittsburgh Sleep Quality Index was used to assess sleep quality and sleep disturbances during winter and summer. A PSQI score of five and higher means a poor sleeping quality. Below five means a good sleeping quality. The index has a sensitivity of 89.6%, and a specificity of 86.5% in differentiating between good and bad sleepers [18].
2.3. Procedure
The subjects were informed about the research on sleep quality and light, and asked to participate. On the first day of the study, the subjects filled out a questionnaire on demographics and aspects of the living situation, such as the normal activity pattern, indoor lighting, and daytime fitness/sleepiness. Then, for five consecutive days, the subjects were asked to keep a sleeping log and an activity log to be filled out every morning. During these five days, participants wore an actigraph, measuring both activity and ambient light level. This protocol was used two times per subject: once in summer (May 2005-June 2005) and once in winter (December 2005-February 2006). Due to time constraints, two subjects were not included in the winter measurement. Two people participated in spring (March) and autumn (October) instead of in winter and summer.
Some subjects were less consistent in wearing the actigraph; therefore, not all subjects have an equal amount of data.
2.4. Analyses
Actigraph data was processed by the Cambridge Neurotechnology Ltd. software package Actiwatch Activity & Sleep Analysis 5, version 5.11. Based on the activity data of the actigraph, the software derives indices of sleep and wakefulness during times when the actigraph is worn. When a subject hardly moves over a longer period of time, this period is assumed as passed by sleeping. Combined with data from the sleeping log, the following indices of sleep were derived: sleep efficiency (percentage of immobility time during time spent in bed at night), sleep latency (time it takes to fall asleep: the difference between the moment the subject lies without moving and the moment indicated to have gone to bed), time initiating sleep (moment one falls asleep), sleeping duration (summation of periods when actigraph registers no activity during times spent in bed), the number of daytime naps (determined by a period of inactivity longer than 20 minutes), the duration of naps and total naptime during the day. Besides variables on sleeping behaviour, the actigraph also recorded light intensity on wrist level. Actiwatch Activity & Sleep Analysis software allows for deriving parameters on light exposure to the actigraph. Parameters used in this study are average daily light exposure, and the amount of time subjects were exposed to illuminances of 1000 / 3000 lx and over per day (as measured on wrist-level).
Statistical analyses were performed on sleep efficiency, sleep latency, sleeping duration, activity, the number of naps, nap duration, the total naptime during the day, the average daily light exposure, and duration of exposure to illuminances of 1000 / 3000 lx and over. Data analysis was carried out using SPSS 12.0.1 for Windows. The critical p-value was set at 0.05. For the analyses of the parameters measured, one-tailed paired samples t-tests were used. Within-subjects analysis were conducted to see whether sleep quality improves by longer exposure to higher light levels, on individual basis. For simple correlations, two-tailed Pearson product-moment correlation coefficients were determined. Additional analyses (mixed models) were conducted to check for confounding factors.
When taking into account all separate data points for each subject, mixed models were used for the statistical analyses. Because not all subjects had an equal amount of measurement days, standard repeated measures analysis was not appropriate. A second motivation for using mixed models (also known as hierarchical models) is that it allows for both slopes and intercepts being estimated separately for each subject. All mixed models used contain a random effect of subject and a repeated measure observation (one observation means one 24 hour period), using a scaled identity covariance type. Furthermore, the fixed effects were analysed using a factorial model including an intercept. An overview of data points, and degrees of freedom in the analyses, are given in Table 1.
3. Materials
3.1. Summer versus winter
3.1.1. Exposure to light
During summer, the average daily light exposure (24 hours) was 658 lx vs. 178 lx in winter. The exposure to light levels over 1 000 lx and over 3 000 lx were significantly longer in summer than in winter (Table 2). Moreover, subjects who were exposed to high ambient light levels in summer also tend to be exposed to high levels in winter (≥1000 lx: r = 0.708, p = 0.033, n = 9; ≥3000 lx: r = 0.823, p = 0.006, n = 9).
Table 2
Table 2. Light exposure duration for summer and winter, and statistical results (p-values) for seasonal differences.
3.1.2. Sleep parameters
Sleep efficiency and sleep latency were both for summer and winter, negatively correlated, indicating that efficient sleep goes hand in hand with short times needed to fall asleep (r=-0.597, p < 0.001, n=98, data points of 14 subjects).
Analysis between-subjects show no significant differences between summer and winter for sleep efficiency, sleep latency, PSQI score, activity, and naps (number of naps, average daily length, and total nap duration). For the results within-subjects, significant correlations between summer and winter were found for sleep efficiency, activity and PSQI. This means that people, who, for instance, sleep efficiently in summer also sleep efficiently in winter. For sleep latency and naps no significant correlation was found between summer and winter.
3.2. Within subjects analyses
3.2.1. Sleep and activity
People who sleep well are more likely to be more active the following day. This is not reflected by the data. For winter, there was a significant effect of sleep latency on daytime activity the day after (t (37) = 2.79, p = 0.008), meaning that the longer it takes to fall asleep the more active the following day. At the same time, there was no significant effect of sleep efficiency on daytime activity the day after (t (37) = -1.84, p = 0.07). In the aggregated data of summer and winter, a similar pattern is found: a significant effect of sleep latency (t (78) = 2.78, p = 0.008), but no effect of sleep efficiency on daytime activity the day after was found (t (78) = -2.19, p = 0.32).
Daytime activity is supposedly larger when one is not too sleepy. Therefore, one might expect long exposure to high light levels to be connected to high daytime activity. One can also argue that, when exposed to high light levels, the participant is most likely outdoors. Being outside, often means that one is active at the same time. However, no significant correlations were found between the data on activity and light exposure, not in winter, nor in summer.
3.2.2. Exposure to light and sleep efficiency
For summer, a significant positive correlation was found for exposure duration to light levels of ≥1000 and ≥3000 lx, and sleep efficiency the following night. Longer exposure to high light levels lead to more efficient sleep (≥1000 lx: t (53) = 3.80, p < 0.001; ≥3000 lx: t (53) = 3.68, p = 0.001). For winter this correlation was not significant (≥1000 lx: t (41) = 1.98, p = 0.054; ≥3000 lx: t (41) = 1.73, p = 0.092) (Fig. 1).
Figure 1
Fig. 1. Scatter plot of sleep efficiency in relation to the exposure duration to light levels of 1 000 lx and over (n = 88) in summer and winter.
3.3. Mixed models analyses
Since exposure to high light levels by being outdoors may go together with increased activity during daytime, the found significant correlation between light exposure and sleep may be confounded by activity.
Mixed models were used with both daytime activity and exposure duration to high light levels as fixed factors. Introducing daytime activity as an explaining factor in the models makes that the models capture the variance due to changes in daytime activity. If the other factor in the model, namely exposure duration still remains to have a significant impact on sleep efficiency, then we know that this effect is not confounded by possible relations with daytime activity.
3.3.1. Exposure to light and sleep efficiency related to activity
For summer, both exposure to light levels of 1 000 lx and over, and 3 000 lx and over, showed a positive effect on sleep efficiency (≥1000 lx: t (47) = 3.51, p = 0.001; ≥3000 lx: t (47)=3.28, p = 0.002), whereas in both analyses, daytime activity had no significant effect on sleep efficiency (≥1000 lx: t (47) = 0.41, p = 0.69; ≥3000 lx: t (47) = 0.11, p=0.92).
Similar patterns were found in winter. The exposure to light levels of 1 000 lx and over showed a significant positive effect on sleep efficiency (t (40) = 2.06, p = 0.046) and a marginal effect of daytime activity was found (t (40) = -1.70, p = 0.098). Exposure duration to light intensities of 3 000 lx and over showed a marginally significant effect on sleep efficiency (t (40) = 1.89, p = 0.07), together with again a marginal effect of daytime activity (t (40) = -1.77, p = 0.084).
3.3.2. Exposure to light and naps
In the aggregated data of summer and winter, exposure to light levels of ≥1000 lx showed a negative effect on the number of naps per day (t (108) = -2.41, p = 0.018) (Fig. 2). No effect was found on nap length.
Figure 2
Fig. 2. Scatter plot of the number of naps in relation to the daily exposure time to light levels of 1 000 lx and over (n = 107).
3.3.3. Sleep and naps
In the same way as sleep and activity were expected to be associated, sleep and daytime napping were hypothesised to be related. People, who sleep badly during the night, may need another nap during the day. Only in winter, relations were found between naps and sleeping. A positive effect was found of sleep efficiency on the number of naps the following day (t (37) = 2.10, p = 0.04).
3.3.4. Activity and naps
Participants that nap during the day are less likely to be active during the same day, as was confirmed by the data. For summer, a significant negative effect of daytime activity was found on the number of naps (t (51) = -5.45, p < 0.001, n = 53), and a negative effect of daytime activity on the total duration of naps per day (t (51) = -4.74, p < 0.001, n = 53). A similar effect of daytime activity on the number of naps was found in the winter situation (t (46) = -7.01, p < 0.001, n = 48).
For winter, daytime activity had a negative effect on the average duration of naps per day, implying that shorter naps go together with more activity during the day (t (46) = -4.06, p < 0.001, n = 48).
In the aggregated data of summer and winter, daytime activity had a negative effect on the number of naps (t (99) = -0.914, p < 0.001, n = 101), as well as it had a negative effect on average nap duration per day (t (99) = -3.20, p < 0.005, n = 101).
4. Discussion and conclusions
4.1. Implications for Theory
The aim of this study was to explore if exposure to high illuminance (> 1000 lx) natural light contributes to a better sleep-wake pattern in older adults. No results were found between summer and winter, probably due to other factors such as the impact of higher ambient temperatures in summer of sleep [31], The larger amount of daylight entering the bedroom late in the evening and early in the morning, or the impact of other indoor climate aspects light CO2-level and temperature [32] The main finding of this study is that a significant effect was found between light exposure and sleep-wake patterns, and more specifically, longer exposure to high light levels is related to higher efficiency of sleep. This result does not change when testing for activity as confounding factor; one might think that not the light exposure, as well the activity is the reason why the sleep efficiency improves. In the aggregated data of summer and winter, no effect was found of sleep efficiency on daytime activity the day after. One would expect someone to be more active when having slept well. Similarly, unexpected patterns were found when studying combinations of sleep and daytime napping. These intuitively rather strange results may be explained from the indirect measurement of sleep variables from actigraph data. Non-efficient sleep corresponds with much activity above the threshold value while lying in bed. If one is just more active, this reflects in bad sleep quality and higher data of activity during the day. This calls for careful interpretation of the relationship between sleep quality measures and light exposure.
Also assumed was that daytime activity is larger when one is not too sleepy. Therefore, one might expect long exposure to high light levels to be connected to high daytime activity. One can also argue that, when exposed to high light levels, the participant is most likely outdoors. Being outside, often means that one is active at the same time. However, no significant correlations were found between the data on activity and light exposure, not in winter, nor in summer. When being exposed to more high light levels> the number of naps that same days are significantly reduced.
The question if light can improve the sleep quality specifically of bad sleepers is still unanswered. Although bad sleepers are not exposed shorter to high light levels than good sleepers, the data prohibits such statement.
4.2. Implications for Practice
Another approach lies within the architecture of the dwellings for the aged. Architects could construct houses for older adults with large windows to the outside world. Special attention should be given to the high eye sensitivity of older adults to differences in luminance. At the same time, we found that subjects wore sunglasses that further reduced the amount of light reaching the retina. In future studies, the effect of sunglasses should be considered in analyses.
The current study is one of the few that analyse the influence of daylight on sleep variables in an unobtrusive, everyday setting. Although the setting and the limited amount of subjects prohibits strong conclusions, evidence is given that there is a positive relation between prolonged exposure to high light levels and efficiency of sleep. In addition, daytime napping appears to happen less frequently when the subject is exposed to high light levels. This indicates that on an individual basis, it might be worthwhile to expose oneself longer a day to more daylight. This could be a healthy and inexpensive solution for sleeping problems, worthwhile of more follow-up research.
4.3. Study limitations
Since this study explored the potential of natural light exposure, its conclusions are limited also due to the number of participant and rather large age range.
The light level on the retina is the factor presumed to influence the sleep-wake cycle. In this study, ambient light level was measured at the wrist level instead of at the eye level. Practical and ethical considerations played a role, for instance, readily available wrist-worn actigraphs versus unpractical measuring goggles or necklaces. Iwata [33] found the following correlation (r = 0.82, n = 28055) (Eq. (1)) between the illumination level at the eye and the wrist, for which differences can be compensated for:
This equation allows for the interpretation of wrist-results to ocular levels. The levels presented in this paper are all Ewrist When considering this equation, a level of 1000 lx on wrist would indicate a level of 518 lx on eye level. In a more recent study [34] a smaller deviation between average eye and wrist exposure was found but strongly depending on the environmental condition people were in, for instance, indoors (27%) and outdoors (11%). Even though sleeping problems can be caused by many different reasons, some may be reduced by exposure to daylight. The sun, a natural source of a high illuminance, provides the light for free. One might argue that in moderate climate zones, older adults do not go outside often enough. Increased exposure to daylight should be encouraged, for instance, by creating awareness among people about the beneficial effects of sunshine.
Acknowledgements
Support from the Light and Health Research Foundation, SOLG, Eindhoven, the Netherlands, is gratefully acknowledged.
Contributions
All authors contributed equally to the writing of the hereby presented paper.
References
- E. J. W. van Someren, Circadian and sleep disturbances in the elderly, Experimental Gerontology 35 (2000) 1229–1237. https://doi.org/10.1016%2Fs0531-5565%2800%2900191-1
- M. P. J. Aarts and A. C. Westerlaken, Field study of visual and biological light conditions of independently-living elderly people, Gerontechnology 4 (2005). https://doi.org/10.4017%2Fgt.2005.04.03.004.00
- C. A. Alessi and J. F. Schnelle, Approach to sleep disorders in the nursing home setting, Sleep Medicine Reviews 4 (2000) 45–56. https://doi.org/10.1053%2Fsmrv.1999.0066
- S. Hattar, R. J. Lucas, N. Mrosovsky, S. Thompson, R. H. Douglas, M. W. Hankins, J. Lem, M. Biel, F. Hofmann, R. G. Foster, and K.-W. Yau, Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice, Nature 424 (2003) 75–81. https://doi.org/10.1038%2Fnature01761
- D. M. Berson, Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock, Science 295 (2002) 1070–1073. https://doi.org/10.1126%2Fscience.1067262
- G. C. Brainard, J. P. Hanifin, J. M. Greeson, B. Byrne, G. Glickman, E. Gerner, and M. D. Rollag, Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor, The Journal of Neuroscience 21 (2001) 6405–6412. https://doi.org/10.1523%2Fjneurosci.21-16-06405.2001
- K. Thapan, J. Arendt, and D. J. Skene, An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans, The Journal of Physiology 535 (2001) 261–267. https://doi.org/10.1111%2Fj.1469-7793.2001.t01-1-00261.x
- B. L. Myers and P. Badia, Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions, Neuroscience & Biobehavioral Reviews 19 (1995) 553–571. https://doi.org/10.1016%2F0149-7634%2895%2900018-6
- D. F. Swaab, E. Fliers, and T. S. Partiman, The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia, Brain Research 342 (1985) 37–44. https://doi.org/10.1016%2F0006-8993%2885%2991350-2
- C. A. Curcio and D. N. Drucker, Retinal ganglion cells in Alzheimer’s disease and aging, Annals of Neurology 33 (1993) 248-57. https://doi.org/10.1002/ana.410330305
- U. Rutishauser, Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions, Trends in Neurosciences 19 (1996) 422–427. https://doi.org/10.1016%2Fs0166-2236%2896%2910041-2
- P. R. Boyce and F. Mcibse, Education: the key to the future of lighting practice, Lighting Research & Technology 38 (2006) 283–291. https://doi.org/10.1177%2F1477153506071330
- D.F. Swaab, Brain aging and Alzheimer’s disease, “Wear and tear” versus “Use it or lose it”. Neurobiology of Aging 12 (1991) 317-324. https://doi.org/10.1016/0197-4580(91)90008-8
- J. van Hoof, M. P. J. Aarts, C. G. Rense, and A. M. C. Schoutens, Ambient bright light in dementia: Effects on behaviour and circadian rhythmicity, Building and Environment 44 (2009) 146–155. https://doi.org/10.1016%2Fj.buildenv.2008.02.005
- M. P. J. Aarts and A. C. Westerlaken, Field study of visual and biological light conditions of independently-living elderly people, Gerontechnology 4 (2005). https://doi.org/10.4017%2Fgt.2005.04.03.004.00
- M. Herljevic, B. Middleton, K. Thapan, and D. Skene, Light-induced melatonin suppression: age-related reduction in response to short wavelength light, Experimental Gerontology 40 (2005) 237–242. https://doi.org/10.1016%2Fj.exger.2004.12.001
- K. Mishima, Diminished Melatonin Secretion in the Elderly Caused by Insufficient Environmental Illumination, Journal of Clinical Endocrinology & Metabolism 86 (2001) 129–134. https://doi.org/10.1210%2Fjc.86.1.129
- C. Kirisoglu and C. Guilleminault, Twenty minutes versus forty-five minutes morning bright light treatment on sleep onset insomnia in elderly subjects, Journal of Psychosomatic Research 56 (2004) 537–542. https://doi.org/10.1016%2Fj.jpsychores.2004.02.005
- M. B. C Aries, M. P. J. Aarts, J. van Hoof, Daylight and health: A review of the evidence and consequences for the built environment, Lighting Research and Technology 47 (2013) 6-27. https://doi.org/10.1177/1477153513509258
- F. Hollwich and B. Dieckhues, The Effect of Natural and Artificial Light via the Eye on the Hormonal and Metabolic Balance of Animal and Man, Ophthalmologica 180 (1980) 188–197. https://doi.org/10.1159%2F000308973
- J. J. Baskett, J. F. Cockrem, and M. A. Todd, Melatonin Levels in Hospitalized Elderly Patients: A Comparison with Community Based Volunteers, Age and Agein 20 (1991) 430–434. https://doi.org/10.1093%2Fageing%2F20.6.430
- K. Obayashi, K. Saeki, J. Iwamoto, N. Okamoto, K. Tomioka, S. Nezu, Y. Ikada, and N. Kurumatani, Positive Effect of Daylight Exposure on Nocturnal Urinary Melatonin Excretion in the Elderly: A Cross-Sectional Analysis of the HEIJO-KYO Study, The Journal of Clinical Endocrinology & Metabolism 97 (2012) 4166–4173. https://doi.org/10.1210%2Fjc.2012-1873
- J. A. Knight, S. Thompson, J. M. Raboud, and B. R. Hoffman, Light and Exercise and Melatonin Production in Women, American Journal of Epidemiology 162 (2005) 1114–1122. https://doi.org/10.1093%2Faje%2Fkwi327
- K. Tsuzuki, I. Mori, T. Sakoi, and Y. Kurokawa, Effects of seasonal illumination and thermal environments on sleep in elderly men, Building and Environment 88 (2015) 82–88. https://doi.org/10.1016%2Fj.buildenv.2014.10.001
- K. Okamoto-Mizuno and K. Tsuzuki, Effects of season on sleep and skin temperature in the elderly, International Journal of Biometeorology 54 (2009) 401–409. https://doi.org/10.1007%2Fs00484-009-0291-7
- M. Calkins, J. G. Szmerekovsky, and S. Biddle, Effect of Increased Time Spent Outdoors on Individuals with Dementia Residing in Nursing Homes, Journal of Housing For the Elderly 21 (2007) 211–228. https://doi.org/10.1300%2Fj081v21n03_11
- J. K. Gammack and J. M. Burke, Natural Light Exposure Improves Subjective Sleep Quality in Nursing Home Residents, Journal of the American Medical Directors Association 10 (2009) 440–441. https://doi.org/10.1016%2Fj.jamda.2009.03.012
- M. Rea, M. Figueiro, and J. Bullough, Circadian photobiology: an emerging framework for lighting practice and research, Lighting Research & Technology 34 (2002) 177–187. https://doi.org/10.1191%2F1365782802lt057oa
- J. M. Torrington and P. R. Tregenza, Lighting for people with dementia, Lighting Research & Technology 39 (2007) 81–97. https://doi.org/10.1177%2F1365782806074484
- D. J. Buysse, C. F. Reynolds, T. H. Monk, S. R. Berman, and D. J. Kupfer, The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research, Psychiatry Research 28 (1989) 193–213. https://doi.org/10.1016%2F0165-1781%2889%2990047-4
- J. van Hoof, Air-conditioned deployable force infrastructure as a strategy to combat sleep deprivation among troops in hot countries, Building Services Engineering Research and Technology 29 (2008) 327–339. https://doi.org/10.1177/0143624408094630
- A. P. Diéguez’, N. Gentile, H. v. Wachenfelt, and M.-C. Duboisba, Daylight Utilization with Light Pipe in Farm Animal Production: A Simulation Approach, Journal of Daylighting 3 (2016) 1-11. http://dx.doi.org/10.15627/jd.2016.1
- T. Iwata, T. Hasebe, and M. Kubota, Study on exposed illuminance in daily life and circadian rhythm. in: Proceedings of the 25th Session of the CIE meeting, 2003, San Diego, CA, USA.
- A. K. Mishra, A. M. van Ruitenbeek, M. G. L. C. Loomans, and H. S. M. Kort, Window/door opening-mediated bedroom ventilation and its impact on sleep quality of healthy, young adults, Indoor Air 28 (2017) 339–351. https://doi.org/10.1111%2Fina.12435
Copyright © 2018 The Author(s). Published by solarlits.com.
 HOME
HOME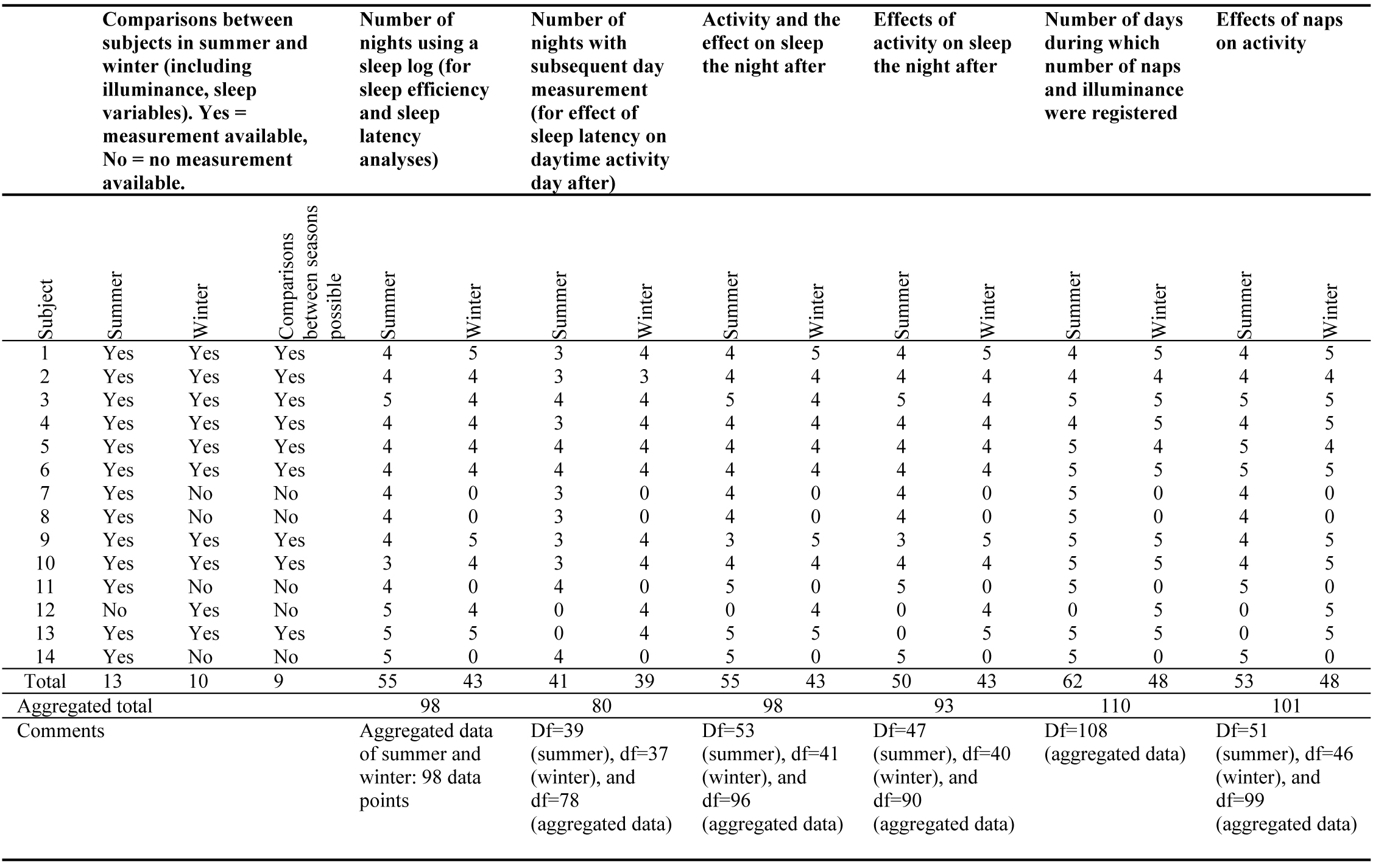 Table 1
Table 1 Table 2
Table 2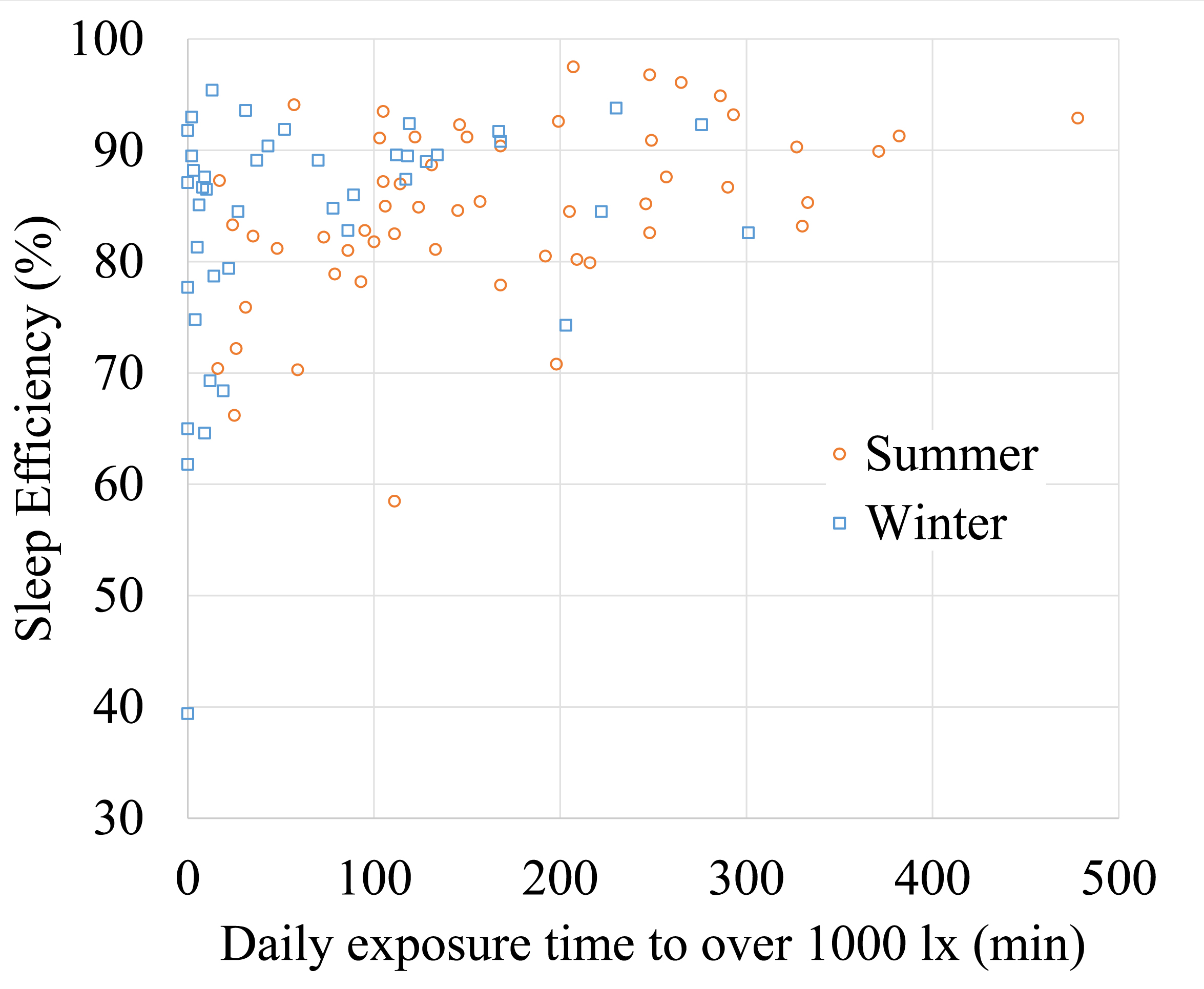 Figure 1
Figure 1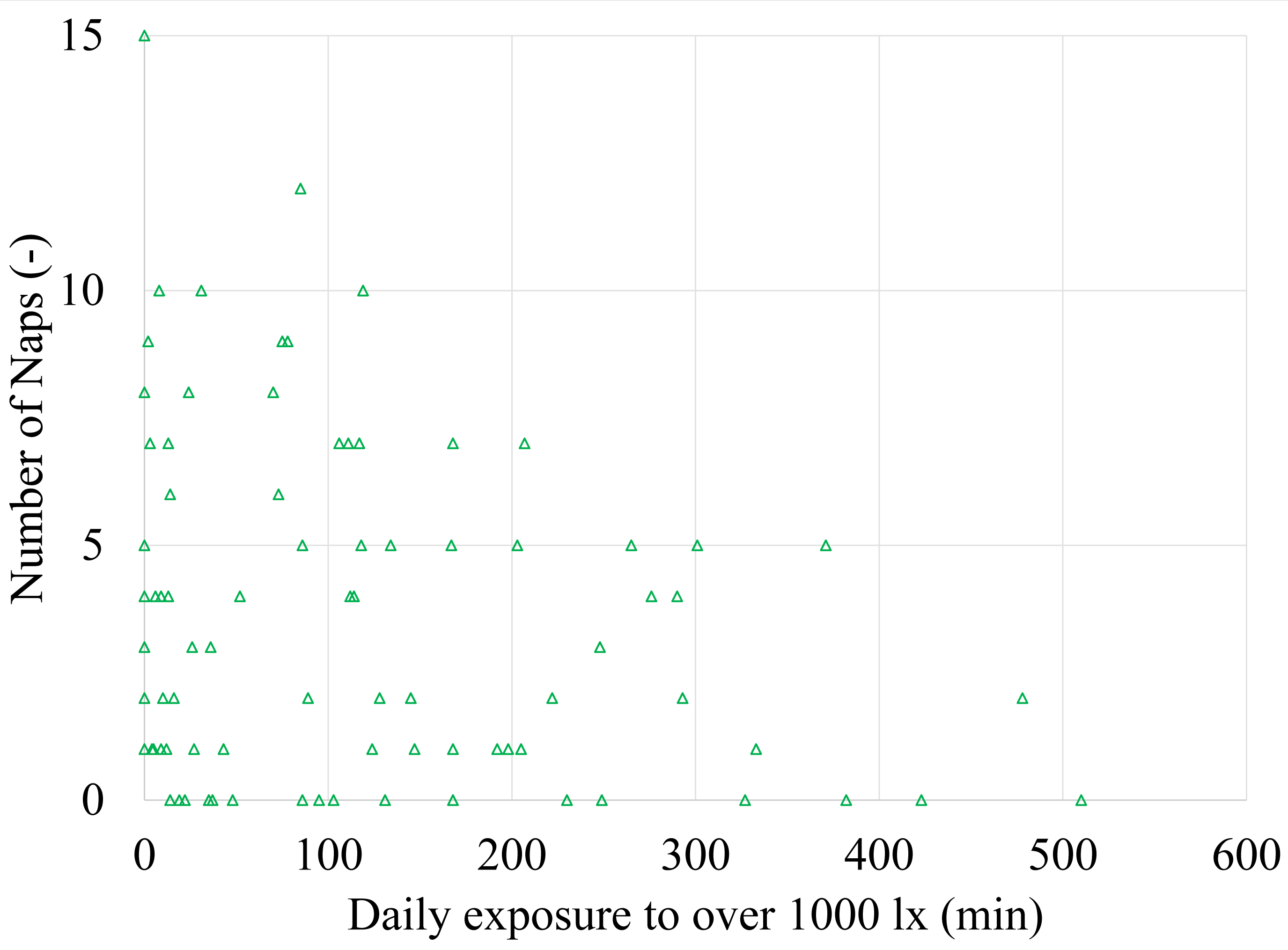 Figure 2
Figure 2


